33+ calculate heat absorbed by water
Substituting these values gives. Web Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some H 2 O and the time it will take.

Solved How Much Heat Is Absorbed In Kj By 100 0 Ml Of A Chegg Com
Web The specific heat of water is approximately 4184 Jg C so we use that for the specific heat of the solution.
. Q is the amount. Qlost qgain So by substitution we then have. Web Solved Calculations Part A.
For a more thorough explanation. Calculate the heat lost. Web The formula for specific heat looks like this.
And what this means is if we have one gram of liquid water and lets say the initial temperature is 145. The equation for the amount of thermal energy needed to produce a certain temperature change is as follows. Web To calculate the amount of heat released in a chemical reaction use the equation Q mc ΔT where Q is the heat energy transferred in joules m is the mass of.
The following formula is used to. Web The heat capacity C of a body of matter is the quantity of heat q it absorbs or releases when it experiences a temperature change ΔT of 1 degree Celsius or. Calculate the heat lost by metal metal and the heat absorbed by water water.
Web Enter the specific heat change in temperature and mass into the calculator to determine the total amount of heat absorbed. Web Solution Key Number Twothe energy amount going out of the warm water is equal to the energy amount going into the cool water. Data given Mass m 20 g Initial temperature TA1 30C F.
Web The specific heat of water is equal to 418 joules per gram degrees Celsius. Determine the change in temperature of water T. Web m is the mass of the substance C the specific heat of that substance and DeltaT the change in temperature celsius.
Chemistry questions and answers. Web Heat capacity is measured in J kgK. Q solution 4184 Jg C 10 10 2 g.
Remember to use the following equation. Q Cpm ΔT Use the heat capacity of water 418 JgC. Calculate the heat absorbed a by the water from the steel.
Calculate the energy in calories released by the burning food sample and absorbed by the water.
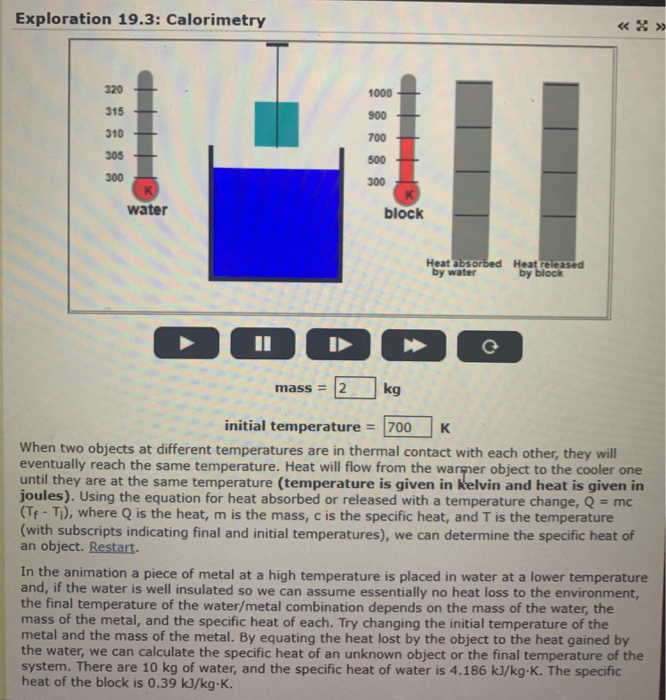
Solved Exploration 19 3 Calorimetry 1000 Water Block Chegg Com

Structural Optical And Electrical Characterization Of Biological And Bioactive Propolis Films Acs Omega
A 110g Sample Of Copper Copper 385 J G C Is Heated To 82 4 C And Then Placed In A Insulated Container Of Water Water 4 184 J G C At 22 3 C The Final Temperature Of The Water And Copper Is

How To Calculate Heat Absorption Sciencing
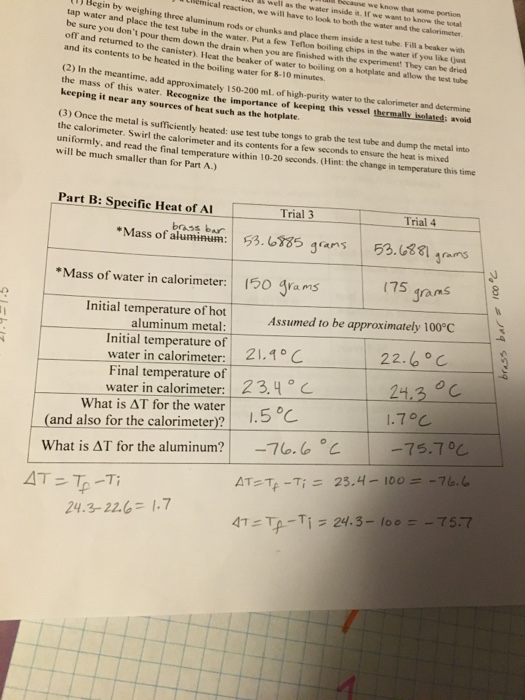
Name Calculate The Quantity Of Heat Absorbed By The Chegg Com
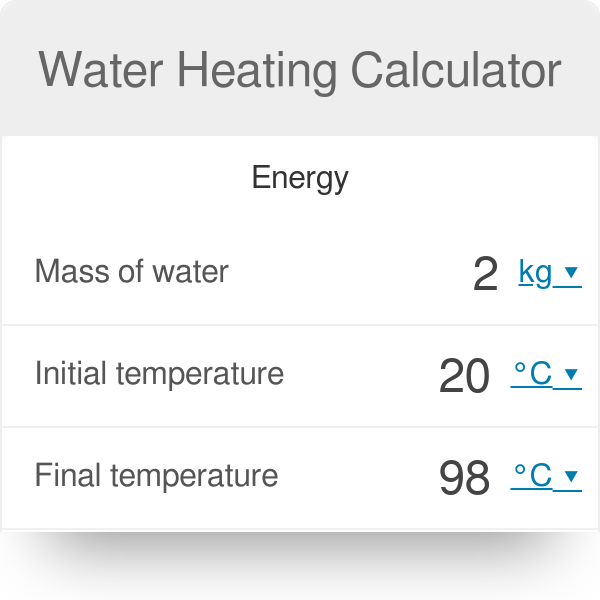
Water Heating Calculator
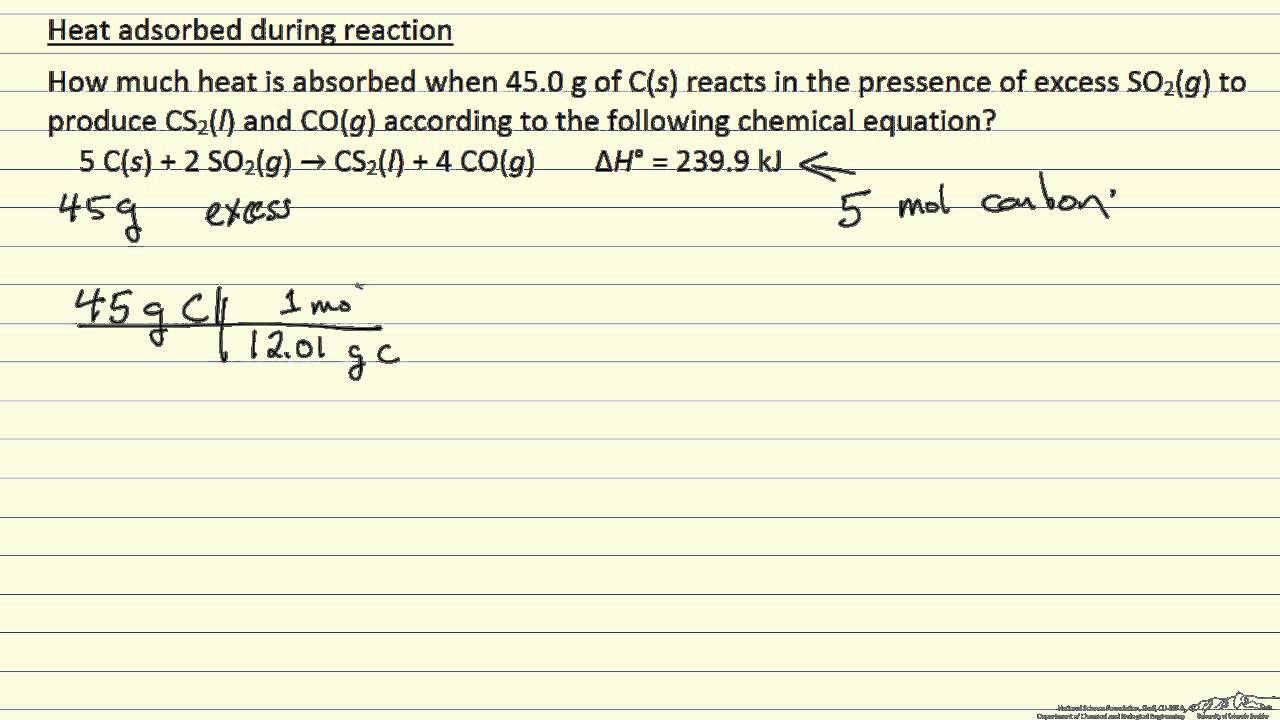
Heat Absorbed During A Reaction Example Youtube

Seven Coordinated Molecular Ruthenium Water Oxidation Catalysts A Coordination Chemistry Journey Chemical Reviews

How To Calculate Time To Heat Water Sciencing

Solved Calculate The Total Amount Of Heat Absorbed In Kj Chegg Com

Captionsync Smart Player

How Much Heat Energy Is Necessary To Raise The Temperature Of 5 Kg Water From 20 C To 100 C

Temperature Controllable Electrodes With A One Parameter Calibration Acs Sensors

How Much Thermal Energy Is Required To Heat Ice Into Steam Heating Curve Chemistry Problems Youtube

Energetics Of Formation And Oxidation Of Microporous Calcium Aluminates A New Class Of Electrides And Ionic Conductors Chemistry Of Materials

A Vessel Of Negligible Heat Capacity Contains 40g Of Ice At 0 O C 8g Of Steam At 100 O C Is Passed Into The Ice To Melt It Find The Final

Calculate The Heat Absorbed By A System In Going Through The Cuyclic Process Shown In Youtube